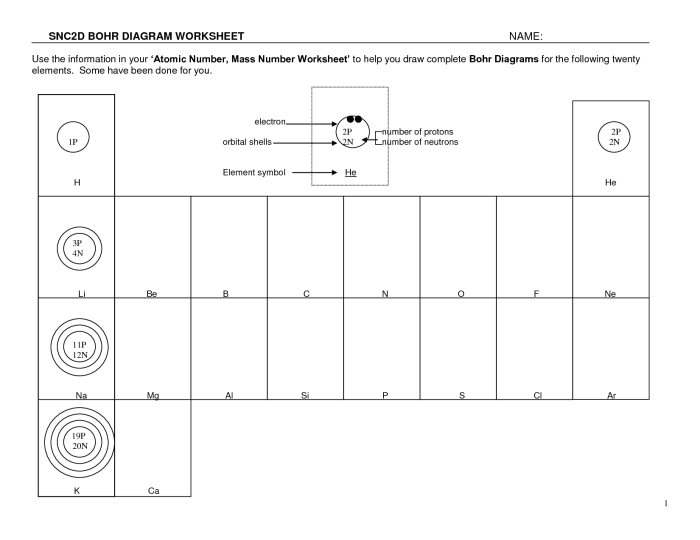
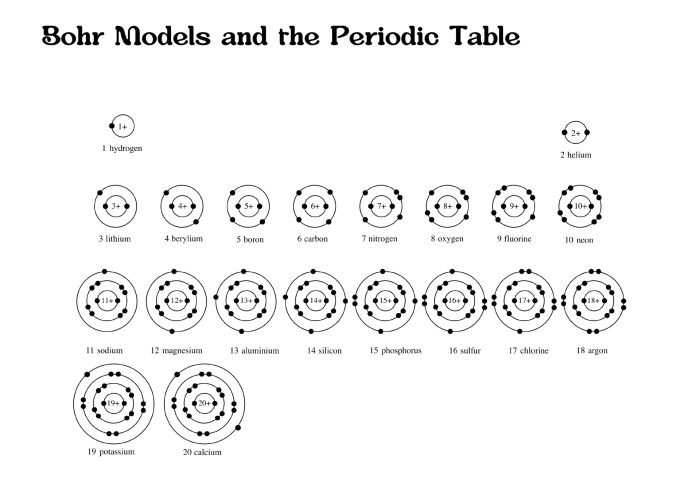
Bohr model diagrams worksheet answers provide a structured framework for students to explore the intricacies of the Bohr model, a seminal theory in understanding atomic structure. This worksheet delves into the fundamental concepts of energy levels, electron configurations, and orbital shapes, empowering students to grasp the complexities of atomic structure and its significance in chemistry.
The Bohr model diagram worksheet serves as a valuable tool for educators to assess student comprehension and reinforce the core principles of atomic structure. By engaging with the worksheet, students actively participate in the learning process, fostering a deeper understanding of the Bohr model and its implications for understanding the behavior of atoms.
Bohr Model Diagrams Worksheet: Bohr Model Diagrams Worksheet Answers
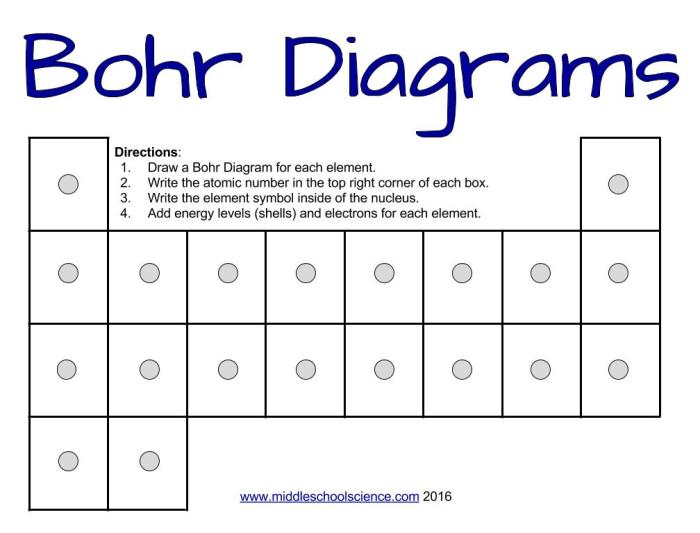
The Bohr model diagram is a visual representation of the Bohr model of the atom. The Bohr model is a simplified model of the atom that describes the arrangement of electrons around the nucleus. The Bohr model diagram can be used to explain a variety of atomic phenomena, such as the emission and absorption of light.
Bohr Model Diagram Worksheet
The following table provides the steps for completing the Bohr model diagram worksheet.
- Draw a circle to represent the nucleus of the atom.
- Draw a series of concentric circles around the nucleus to represent the energy levels of the atom.
- Place electrons in the energy levels according to the following rules:
- Each energy level can hold a maximum of 2n2electrons, where n is the energy level number.
- Electrons must occupy the lowest energy level available.
- Electrons must be paired in the energy levels.
- Label the energy levels and the electrons.
Bohr Model Diagram Answers
The following table provides the correct answers for the Bohr model diagram worksheet.
| Energy Level | Number of Electrons | Electron Configuration | Orbital Shape |
|---|---|---|---|
| 1 | 2 | 1s2 | Spherical |
| 2 | 8 | 2s22p6 | Spherical and dumbbell |
| 3 | 18 | 3s23p63d10 | Spherical, dumbbell, and cloverleaf |
| 4 | 32 | 4s24p64d104f14 | Spherical, dumbbell, cloverleaf, and complex |
Additional Practice, Bohr model diagrams worksheet answers
The following are a set of practice problems for students to complete.
- Draw a Bohr model diagram for the element oxygen.
- Explain why the electrons in the outermost energy level of an atom are the most reactive.
- Predict the chemical properties of an element based on its Bohr model diagram.
Questions and Answers
What is the purpose of the Bohr model diagram worksheet?
The Bohr model diagram worksheet is designed to guide students in understanding the structure of atoms and the distribution of electrons within energy levels, providing a visual representation of the Bohr model.
How do I determine the number of electrons in each energy level?
The number of electrons in each energy level is determined by the formula 2n², where n represents the energy level. For example, the first energy level (n=1) can hold up to 2 electrons, while the second energy level (n=2) can hold up to 8 electrons.
What is the significance of electron configuration in the Bohr model?
Electron configuration describes the arrangement of electrons in different energy levels and orbitals. It helps determine the chemical properties and behavior of an element.